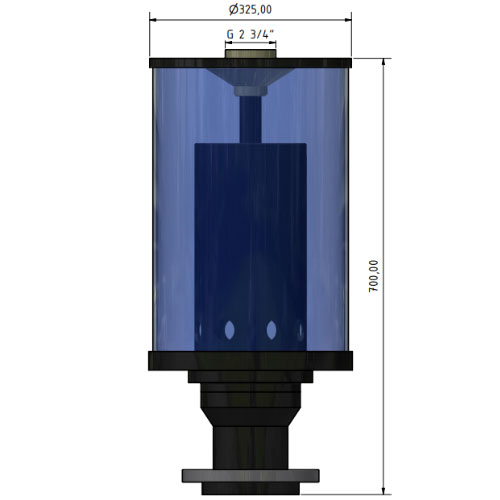
CO2 absorber type SDA315-FL-100 with PVC U loose flange DN 100, d 110 mm
delivery time: 1 week
|
| - CO2 absorber for use in medical technology and clean room applications - CO2 is absorbed and absorbed through the granules - Sterile filter cartridge for micro solids 0.10, 0.20 and 0.3 micron optimally available - Optimally 3 sterile filter cartridge |
|
|
||||||||||||||||||||||||||||||
Information:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Effective CO2 Absorption for a Sustainable Future! |
|
CO2 absorption using lime is a chemical process that involves removing carbon dioxide (CO2) from a gas stream by reacting it with lime (calcium hydroxide). This process is commonly employed in industrial facilities to reduce CO2 emissions and mitigate environmental impact. The CO2 absorption process with lime can be described as follows:- Lime Introduction: Lime in the form of powder or granules is introduced into an absorber or reactor. The absorber is connected to a gas stream containing CO2. - Reaction with CO2: The CO2 in the gas stream reacts with lime, forming calcium carbonate (limestone). The reaction can be represented by the following equation: CO2 + Ca(OH)2 → CaCO3 + H2O. - Formation of Calcium Carbonate: The resulting calcium carbonate is formed as a solid substance and accumulates in the absorber. It can exist as limestone or in other forms. - Removal of Absorbed CO2: To ensure continuous CO2 absorption, the deposited limestone is periodically removed from the absorber. This can be done through mechanical methods such as scraping or chipping. - Regeneration of Lime: The separated limestone undergoes a regeneration stage where CO2 is separated from calcium carbonate. This results in the formation of calcium oxide (quicklime) and CO2. The quicklime can be reintroduced into the absorber to continue the process. The CO2 absorption process using lime offers an effective method for reducing CO2 emissions in various industries such as power plants, cement manufacturing, and other high-emission facilities. By reacting lime with CO2, the greenhouse gas is removed from the gas stream and bound in a solid form, leading to a reduction in CO2 burden. |
Optimize your shopping experience with our detailed delivery information |
Partnership at the highest level. |
|
|
|
|
|
|
|